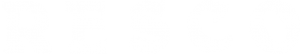INOSINE
Abstract
Inosine pranobex (Isoprinosine®) is an immunomodulatory drug approved in several countries for the treatment of viral infections. This study compared the efficacy and safety of inosine pranobex versus placebo in subjects with clinically diagnosed influenza- like illness, including subjects with laboratory-confirmed acute respiratory viral infections. Subgroup analyses evaluated the efficacy of inosine pranobex compared to placebo in otherwise healthy (without related ongoing disease) subjects that were less than 50 years of age and healthy subjects that were at least 50 years of age. The effect of body mass index (BMI) was evaluated in subjects less than 50 years of age.
Background
Acute respiratory infection is a serious infection that is responsible for approximately 3.9 million deaths per year and is one of the leading causes of morbidity and mortality worldwide [1]. Acute respiratory infections are categorized as either upper or lower respiratory infections and are caused by well-recognised viral pathogens, including but not limited to influenza virus (types A and B), parainfluenza virus, respiratory syncytial virus (RSV), metapneumovirus (types A and B), coronavirus, rhinovirus, enterovirus, reovirus, bocavirus, and adenovirus, and bacterial pathogens, primarily Streptococcus.
Influenza is an acute viral respiratory infection that affects 5 % to 15 % of the global adult population per year and results in approximately 0.25 to 0.5 million deaths and 3 to 5 million cases of severe illness worldwide annually [4, 5]. Influenza-like illnesses (ILI) are considered a subset of acute respiratory infections and result in the sudden onset of symptoms such as fever (body temperature greater than 38 °C), cough, and sore throat in patients [5]. Physicians have difficulty with the treatment of ILI because determining the aetiology is generally not possible solely on a clinical basis. Current pharmacological interventions for the prevention and treatment of respiratory viral infections are primarily limited to vaccines and antivirals for influenza. Available antiviral treatments for influenza infection are M2 ion channel inhibitors (eg, amantadine andrimantadine) and neuraminidase inhibitors (eg, oseltamivir and zanamivir) [6]. If the infection is caused by bacterial pathogens, treatment can involve antibiotics and also medications that provide symptomatic relief. However, in case of viral aetiology, only medication for symptomatic treatment can be provided. [7]. Vaccination for seasonal influenza remains one of the standard approaches for prevention of the disease. However, immunisation rates for seasonal influenza remain low in many European countries even though the influenza vaccine is part of the national immunisation schedule in these countries [8]. Moreover, the vaccination is eective only when most of the circulating influenza viruses in a given season are similar to the viruses that were included in the influenza vaccine. The vaccine does not oer any clinical benet against other pathogens that circulate during a season [9]. Inappropriate use of antibiotics for the treatment of acute respiratory infections has been observed during winter months, even though the majority of infections are caused by viral pathogens and are self-limiting. This practice may result in side effects and the development of antibiotic resistance in pathogens as well as an increased cost burden for the healthcare system [3, 10, 11]. Inosine pranobex (Isoprinosine®), a combination of the p-acetamidobenzoate salt of N,N–dimethylamino-2-propanol and inosine in a 3:1 molar ratio, is an immunomodulatory antiviral drug that has been licensed since 1971 in several countries worldwide for the treatment of viral infections [2, 12]. Inosine pranobex stimulates a nonspecific immune response that is independent of the specific viral antigen responsible for the ILI. In clinical studies, inosine pranobex has been shown to induce a type 1 T helper cell-type response in mitogen- or antigen-activated cells, and this response initiates T-lymphocyte maturation and differentiation and potentiates induced lymphoproliferative responses (13–15). Similarly, the drug modulates T-lymphocyte and natural killer cell cytotoxicity and CD8+ suppressor and CD4 + -helper cell functions and increases the number of immunoglobulin G and complement surface markers (14, 15). Inosine pranobex also increases cytokine interleukin (IL)-1 production and IL-2 production and upregulates the expression of the IL- 2 receptor in vitro [13, 14]. The safety prole of inosine pranobexhas been established through clinical trials for several indications and populations [2, 14–16]. A rapid increase in the number of mononuclear cells after the first dose of inosine pranobex was observed in 75 % of the subjects, and this increase was consistent with clinical observations of rapid resolution of common cold symptoms [15, 17].